Background
The prognosis of pts with TKI-resistant or T315I-mutated CML has markedly improved since the introduction of third-generation TKIs (3G-TKIs). However, there are limited data on the HRQOL of pts receiving 3G-TKI therapy. Olverembatinib is a novel 3G-TKI, which has shown efficacy and safety in pts with TKI-resistant chronic- or accelerated-phase CML (CP-CML/AP-CML). Here, we report the HRQOL of pts with TKI-resistant CML receiving olverembatinib treatment and associated variables.
Method
The European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) questionnaire was used to assess the HRQOL of pts with CML enrolled in phase 1/2 clinical trials on olverembatinib conducted in Peking University People's Hospital. HRQOL was evaluated at baseline and 3, 6, 12, 24, and 36 months. All scales and single-item measures were standardized and ranged from 0 to 100. A high score in functional scales and global health/QOL represented improved function or QOL. A high score in symptom scales represented worse symptomatology or problems. We measured score changes over time and changes in scores (CIS) at month 36 compared to baseline. Comparison of means was performed using a Student paired t-test. Mixed-effects models were used to identify which variables were associated with improvements in functional scales and reductions in symptom scales of the EORTC QLQ-C30.
Results
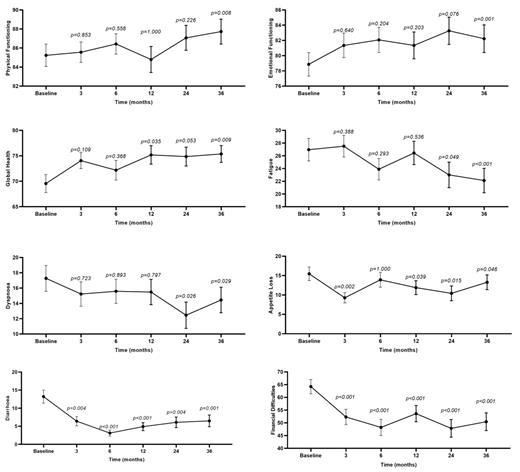
Overall, 164 Chinese pts with CML (male, n = 106 [65%]) were enrolled and completed the questionnaire at the start of the study. A total of 132 (80%) pts had CP-CML and 32 AP-CML. The median (range) age was 43 (20-74) years. A total of 54 (33%) pts received ≥ 3 types of TKIs before starting olverembatinib therapy. The top three symptom burdens reported by pts at baseline were financial difficulties (64.2 ± 36.1), fatigue (27.0 ± 22.7), and insomnia (20.3 ± 27.3). During the 3-year follow-up, 119 (75%) pts achieved major cytogenetic response (MCyR) and 113 (69%) completed the questionnaire at 36 months. Among the 15 global health status items, functional and symptom scales significantly improved over time, including global health/QOL (CIS, +7.1; p < 0.01); physical functioning (CIS, +4.0; p < 0.01); emotional functioning (CIS, +6.0; p < 0.01); fatigue (CIS, -7.8; p < 0.001); dyspnea (CIS, -5.5; p < 0.05); appetite loss (CIS, -5.5; p < 0.05); diarrhea (CIS, -11.9; p < 0.001); and financial difficulties (CIS, -19.6; p < 0.001) (Figure 1). No scale deteriorated statistically significantly over time. Mixed-effects models that were used to identify variables associated with improvements in functional scales and reductions in symptom scales revealed that greater respective improvements in physical functioning and fatigue scales were significantly associated with younger (< 40 years) age ( p= 0.018; p= 0.029) and achievement of MCyR ( p= 0.008; p= 0.050); in the appetite loss scale, younger (< 40 years) age ( p = 0.001); in the emotional functioning scale, male sex ( p= 0.012); in the global health scale, CP-CML status at baseline ( p= 0.048); in the dyspnea scale, female sex ( p< 0.001) and CP-CML status at baseline ( p= 0.044); and in the diarrhea scale, achievement of MCyR ( p= 0.002).
Conclusions
The HRQOL of pts with TKI-resistant CML significantly improved during olverembatinib therapy. Younger age, being in CP-CML compared to AP-CML at baseline, and achieving MCyR on treatment were the common variables associated with improved HRQOL.
Figure 1. HRQOL profile
Disclosures
Men:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal